Abstract
Background: Chimeric antigen receptor (CAR) T-cell immunotherapy is a paradigm shifting treatment option for multiple refractory hematologic malignancies. Immune effector cell-associated neurotoxicity syndrome (ICANS) is a serious adverse effect (AE) associated with CAR T-cell therapy. To date, corticosteroids remain the cornerstone treatment of ICANS. Recently, limited clinical data have suggested that the addition of anakinra may be beneficial in the treatment of corticosteroid refractory severe ICANS.
Methods: We performed a retrospective chart review of patients with a diagnosis of refractory B cell lymphoma treated with CART-cell therapy at the University of Arizona Cancer Center (UACC), who subsequently developed severe ICANS (grade 3-4). We report five cases in which anakinra (at the dose of 100 mg daily) was used as an adjunct therapy to high dose steroid treatment in managing severe ICANS.
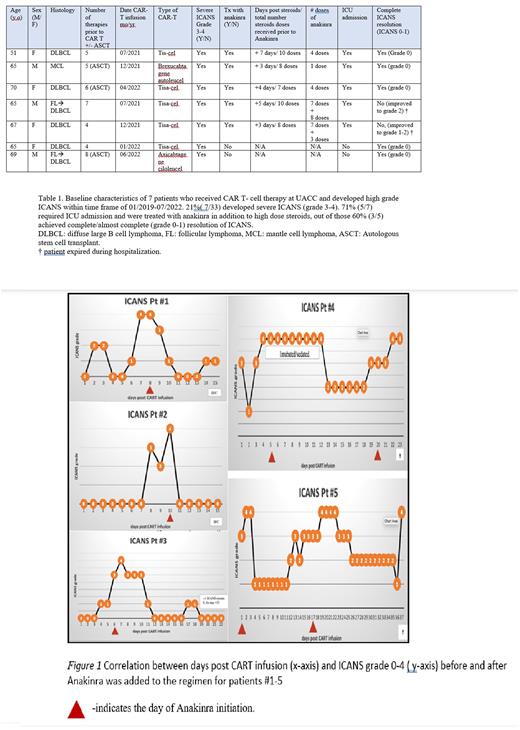
Results: A total of 33 patients received CART-cell therapy at UACC from 01/2019-07/2022. The incidence of severe ICANS was 21% (7/33). Baseline characteristics of 7 patients who developed high grade ICANS are summarized in Table 1. The median age was 65 (range 51-70). 5 patients were treated with Tisagenlecleucel, 1 with Axicabtagene ciloleucel and 1 with Brexucabtagene autoleucel CAR-T cell therapy. The median time to onset of severe ICANS was 6 days (range 1-13 days). Two out of seven patients (29%) developed grade 3 ICANS which resolved quickly with corticosteroids in 1-3 days. Five out of seven patients (71%) developed grade 4 ICANS, requiring ICU admission and high dose corticosteroids. Patients received at least 3 days of high dose corticosteroids with methylprednisolone 500 mg IV twice a day before anakinra was added for steroid refractory ICANS. On average, patients received ~ 9 doses of corticosteroids prior to anakinra administration. After addition of anakinra, three patients had a complete resolution of ICANS, two patients had partial improvement in their ICANS (grade 1-2).
For the three patients with complete resolution of ICANS, the median number of anakinra doses were 4, median duration of severe ICANS (post-anakinra) was 3 days and the median time in ICU was 3 days. Clinical course was complicated for the other two patients with acute hypoxic respiratory failure in one patient and E. coli bacteremia, fulminant pseudomembranous colitis s/p colectomy, and septic shock in the second patient leading to death on days +23 and +40 respectively.
Conclusion: Although limited in number, our analysis suggests anakinra may be effective and safe in steroid refractory ICANS. ICANS resolved completely in three out of five patients following administration of anakinra who were refractory to high dose corticosteroids, and partially improved to grade 1-2 in two patients. No significant side effects attributed to anakinra were observed. Future randomized controlled studies are needed to better understand the overall efficacy and the ideal timeline for administration.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal